Effects of odevixibat vs placebo on hepatic biochemical parameters and liver adverse events in patients with PFIC: Data from the PEDFIC 1 study
Tassos Grammatikopoulos1, Reha Artan1, Pier Luigi Calvo1, Piotr Czubkowski1, Buket Dalgic1, Lorenzo D’Antiga1, Özlem Durmaz1, Emmanuel Gonzalès1, Girish Gupte1, Winita Hardikar1, Binita M. Kamath1, Florence Lacaille1, Cara L. Mack1, Patrick McKiernan1, Hasan Özen1, Sanjay R. Rajwal1, Mathias Ruiz1, Mohammad Shagrani1, Eyal Shteyer1, Nisreen Soufi1, Mary Elizabeth Tessier1, Wendy L. van der Woerd1, Fatine Elaraki1, Tao Gu1, Michelle Hammerschmidt1, Alejandra Ramirez-Santiago1, Danielle Dray1, Terese Wallefors1, Douglas Silveira1, Henkjan J. Verkade1.
1Number of associated affiliations not allowed; Please see Word draft provided to info@split2025.org , Parsippany, NJ, United States
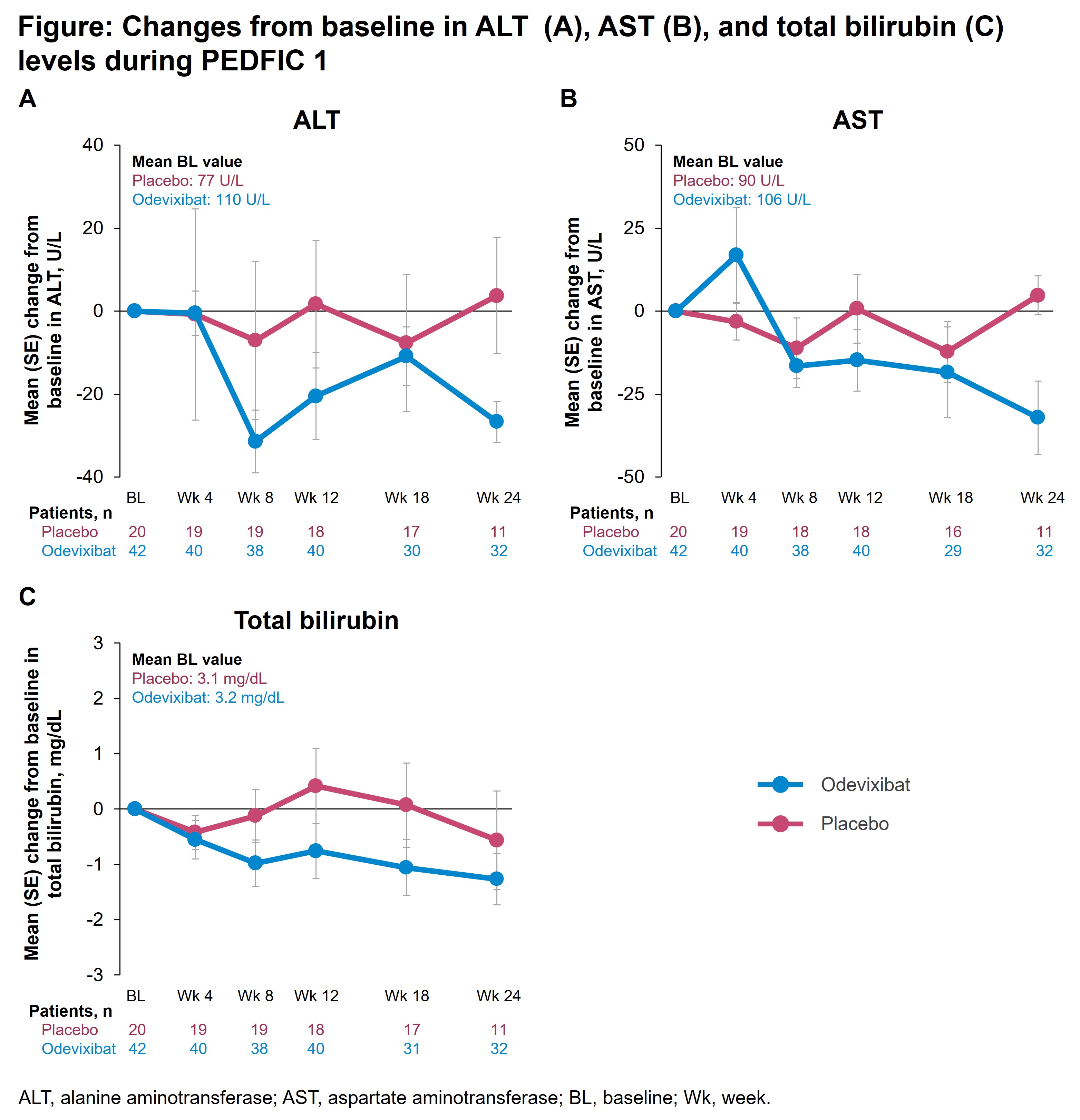
Introduction: In the 24-week PEDFIC 1 study (NCT03566238), odevixibat significantly reduced serum bile acids and improved pruritus in patients with progressive familial intrahepatic cholestasis (PFIC). To better understand odevixibat’s impact on liver function, we analyzed data on hepatic biochemical parameters and liver events from PEDFIC 1.
Method: Eligible children received placebo or odevixibat. Assessments included hepatic biochemical parameters (alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin [TB]), liver-related treatment-emergent adverse events (TEAEs), and elevations in hepatic parameters evaluated using the modified evaluation for drug-induced severe hepatotoxicity (eDISH) approach. A Data Safety Monitoring Board (DSMB) reviewed prespecified hepatic events.
Results: In PEDFIC 1, 62 patients were randomized (n=20, placebo; n=42, odevixibat). Mean changes in ALT, AST, and TB levels are presented (Figure). Overall, 4/20 (20%) patients receiving placebo and 11/42 (26%) receiving odevixibat had liver-related TEAEs; the most common with odevixibat were ALT increased (n=6 [14%]; placebo, n=1 [5%]) and blood bilirubin increased (n=5 [12%]; placebo, n=2 [5%]). Potentially clinically significant ALT or AST elevations (ie, >3× baseline levels) were observed in 4 odevixibat-treated patients, but no concurrent TB elevations were observed. There were no confirmed cases of drug-induced liver injury (DILI); liver-related events were assessed as related to patients’ underlying disease or other causes. No patients developed liver decompensation.
Conclusion: Following odevixibat treatment in patients with PFIC, mean transaminase and TB levels decreased, whereas only minimal changes were observed with placebo. DSMB review did not identify any confirmed cases of DILI or any liver adverse events related to study drug.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00