ASSERT-EXT: Final data from an open-label, phase 3 study of odevixibat in patients with Alagille syndrome
Nadia Ovchinsky1, Madeleine Aumar1, Alastair Baker1, Philip Bufler1, Mara Cananzi1, Piotr Czubkowski1, Özlem Durmaz1, Ryan Fischer1, Giuseppe Indolfi1, Wikrom W. Karnsakul1, Florence Lacaille1, Way S. Lee1, Giuseppe Maggiore (13), Mathias Ruiz1, Etienne Sokal1, Ekkehard Sturm1, Wendy L. van der Woerd1, Andrew Wehrman1, Christof Maucksch1, Judy Zhu1, Alejandra Ramirez-Santiago1, Danielle Dray1, Henkjan J. Verkade1.
1Number of associated affiliations not allowed; Please see Word draft provided to info@split2025.org , Parsippany, NJ, United States
13 Hepatology and Liver Transplantation Unit, Bambino Gesù Children's Hospital IRCCS, Rome, Italy;.
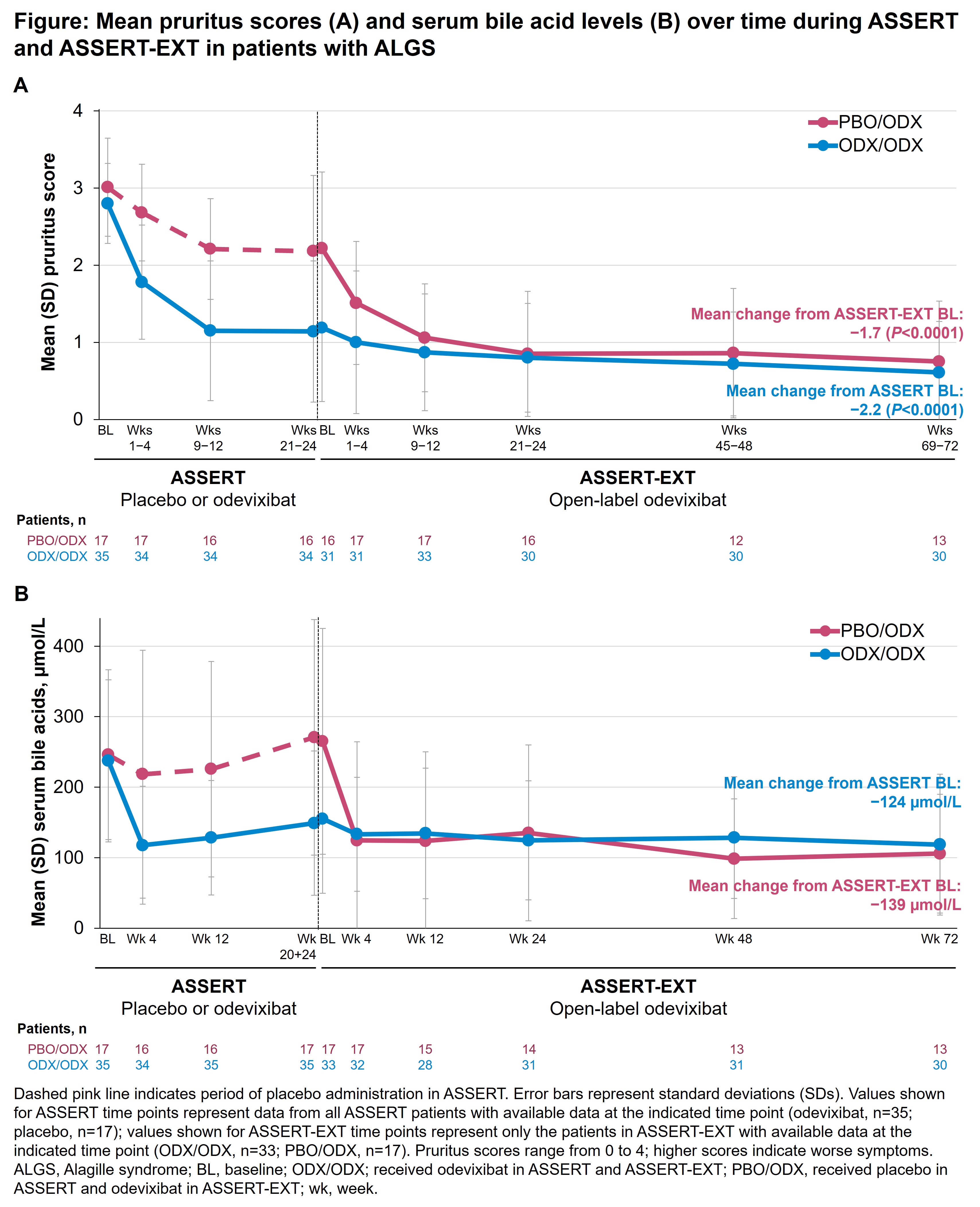
Introduction: In the 24-week, phase 3 ASSERT study (NCT04674761), odevixibat significantly lowered pruritus and serum bile acids (sBAs) in patients with Alagille syndrome (ALGS). Here, we report long-term data on odevixibat efficacy and safety from the 72-week, open-label ASSERT-EXT study (NCT05035030).
Method: Patients who completed ASSERT were eligible to enter ASSERT-EXT; all patients in ASSERT-EXT received odevixibat 120 μg/kg/day. Outcomes included change in pruritus score, change in sBAs, and treatment-emergent adverse events (TEAEs). Results were evaluated in patients who received odevixibat in ASSERT and ASSERT-EXT (ODX/ODX) and patients who received placebo in ASSERT and odevixibat in ASSERT-EXT (PBO/ODX); baseline was pre‑odevixibat (ASSERT baseline for ODX/ODX and ASSERT-EXT baseline for PBO/ODX).
Results: Of 52 patients who completed ASSERT, 50 (median [range] age, 5.5 [1.0−15.9] years) entered ASSERT‑EXT (ODX/ODX, n=33; PBO/ODX, n=17). Mean (SD) changes in pruritus score from baseline to weeks 69−72 of ASSERT-EXT were −2.2 (0.9) points (ODX/ODX; P<0.0001) and −1.7 (0.8) points (PBO/ODX; P<0.0001; Figure A). Mean (SD) changes in sBAs from baseline to week 72 of ASSERT-EXT were −124 (136) µmol/L (ODX/ODX) and −139 (101) µmol/L (PBO/ODX; Figure B). The most common drug-related TEAE was diarrhea (ODX/ODX: 2/33 [6%]; PBO/ODX: 3/17 [18%]).
Conclusion: Odevixibat significantly improved pruritus and reduced sBA levels in patients with ALGS; these improvements were sustained for up to 96 weeks in patients who remained on odevixibat. The safety profile of odevixibat in ASSERT-EXT was consistent with that in ASSERT. These robust long-term data demonstrate durable efficacy and tolerability of odevixibat in patients with ALGS.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00