Open-label study of odevixibat safety and tolerability in infants aged <12 months with Alagille syndrome: Clinical study design
Nadia Ovchinsky1, Quanhong Ni2, Roshawn Watson2, Praneeta Nagraj2, Danielle Dray2, Alejandra Ramirez-Santiago2.
1Pediatric Gastroenterology and Hepatology, Hassenfeld Children’s Hospital at NYU Langone, New York, NY, United States; 2Ipsen, Cambridge, MA, United States
Introduction: In the phase 3, 24-week ASSERT study (NCT04674761), odevixibat was efficacious in reducing pruritus and serum bile acids in children aged >12 months with Alagille syndrome (ALGS). ASSERT-EXT (NCT05035030) is an ongoing phase 3, open-label study with 2 cohorts: cohort 1 is a 72-week extension study in patients who completed ASSERT, and cohort 2 is a 12-week study evaluating the safety, pharmacokinetics, and pharmacodynamics of odevixibat in infants aged <12 months with ALGS. Here, we describe the study design of cohort 2.
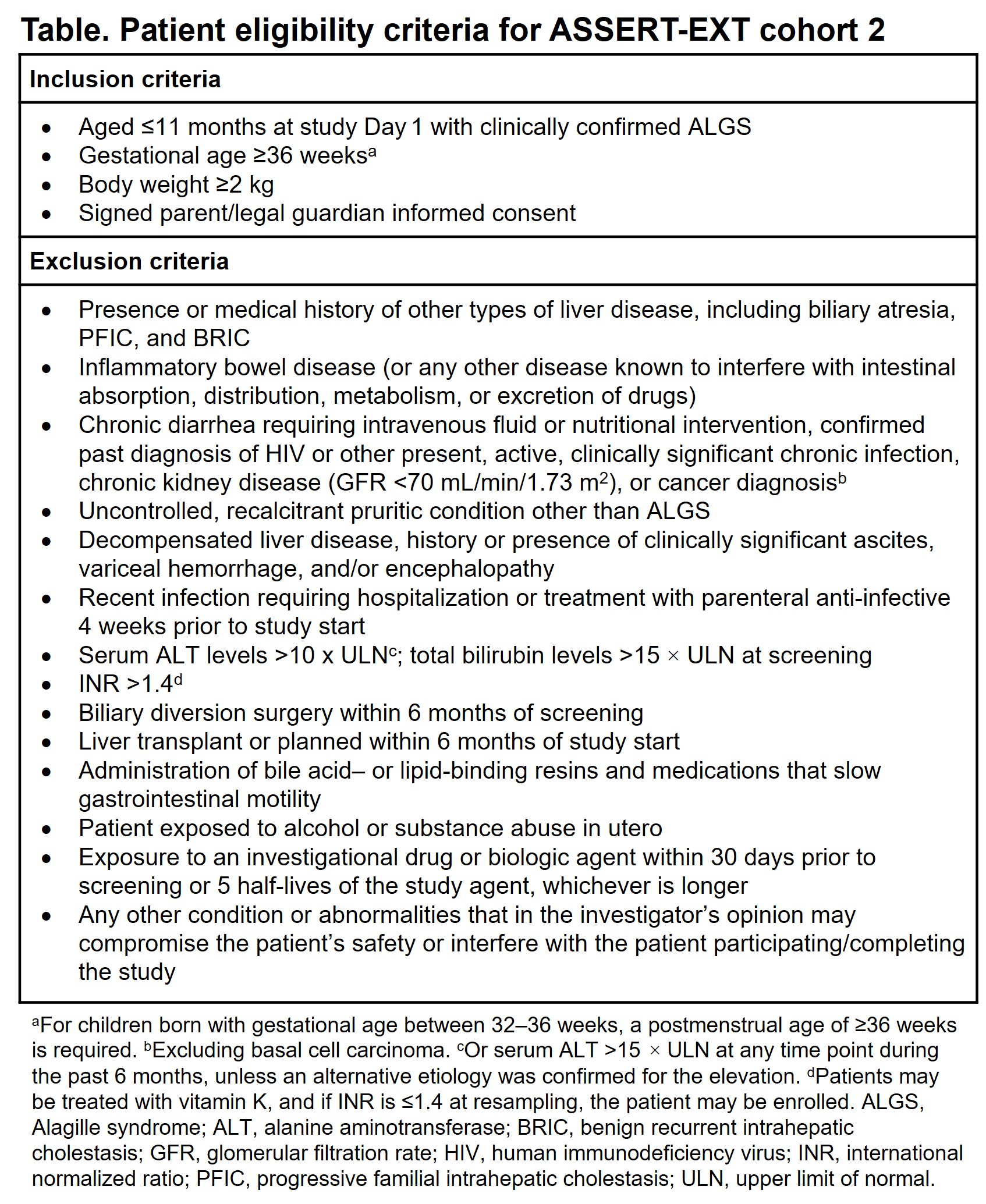
Method: Cohort 2 is expected to enroll approximately 10 infants with ALGS aged ≤11 months. Inclusion and exclusion criteria are shown in the Table. Eligible patients will receive odevixibat 120 µg/kg once daily for 12 weeks. For administration in infants, odevixibat capsules can be opened and the contents sprinkled onto soft food or mixed into infant formula or breast milk. The primary objective of cohort 2 is to evaluate odevixibat safety and tolerability, which will be assessed by analyzing treatment-emergent adverse events and changes in physical examination, concomitant medications, vital signs, laboratory tests, and fat-soluble vitamin levels. Secondary objectives are to evaluate odevixibat pharmacokinetics at Day 1 and weeks 4, 8, and 12, and changes in serum bile acid levels from baseline to Week 12.
Results: Not applicable
Conclusion: Odevixibat has a well-established efficacy and tolerability profile in patients aged >12 months with ALGS. Results from cohort 2 of ASSERT-EXT will help to determine the safety and tolerability of odevixibat in patients aged <12 months with ALGS.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00