Prospective validation of a novel algorithm for liver transplantation in children with Langerhans cell histiocytosis
Jagadeesh Menon1, Naresh Shanmugam1, Ashwin Rammohan2, Mukul Vij3, Meena Sivasankaran4, Kumar Palaniappan2, Mohamed Rela2.
1Pediatric gastroenterology and hepatology , Dr Rela Institute & Medical Centre, Chennai, India; 2Hepatobiliary surgery & liver transplantation, Dr Rela Institute & Medical Centre, Chennai, India; 3Histopathology, Dr Rela Institute & Medical Centre, Chennai, India; 4Pediatric hematology & oncology, Dr Rela Institute & Medical Centre, Chennai, India
Background : We previously came up with a novel algorithm to treat end stage liver disease (ESLD) in children with Langerhans cell histiocytosis (LCH). In the current analysis we try to validate this prospectively in the subsequent cohort of patients with ESLD secondary to LCH.
Methods : All patients with LCH undergoing a liver transplant(LT) from September 2022 till December 2024 were enrolled. Clinical profile, chemotherapy protocols, LT outcomes were described.
Results : 10 (5%) of 206 LT in the aforementioned period were performed for LCH(Fig1)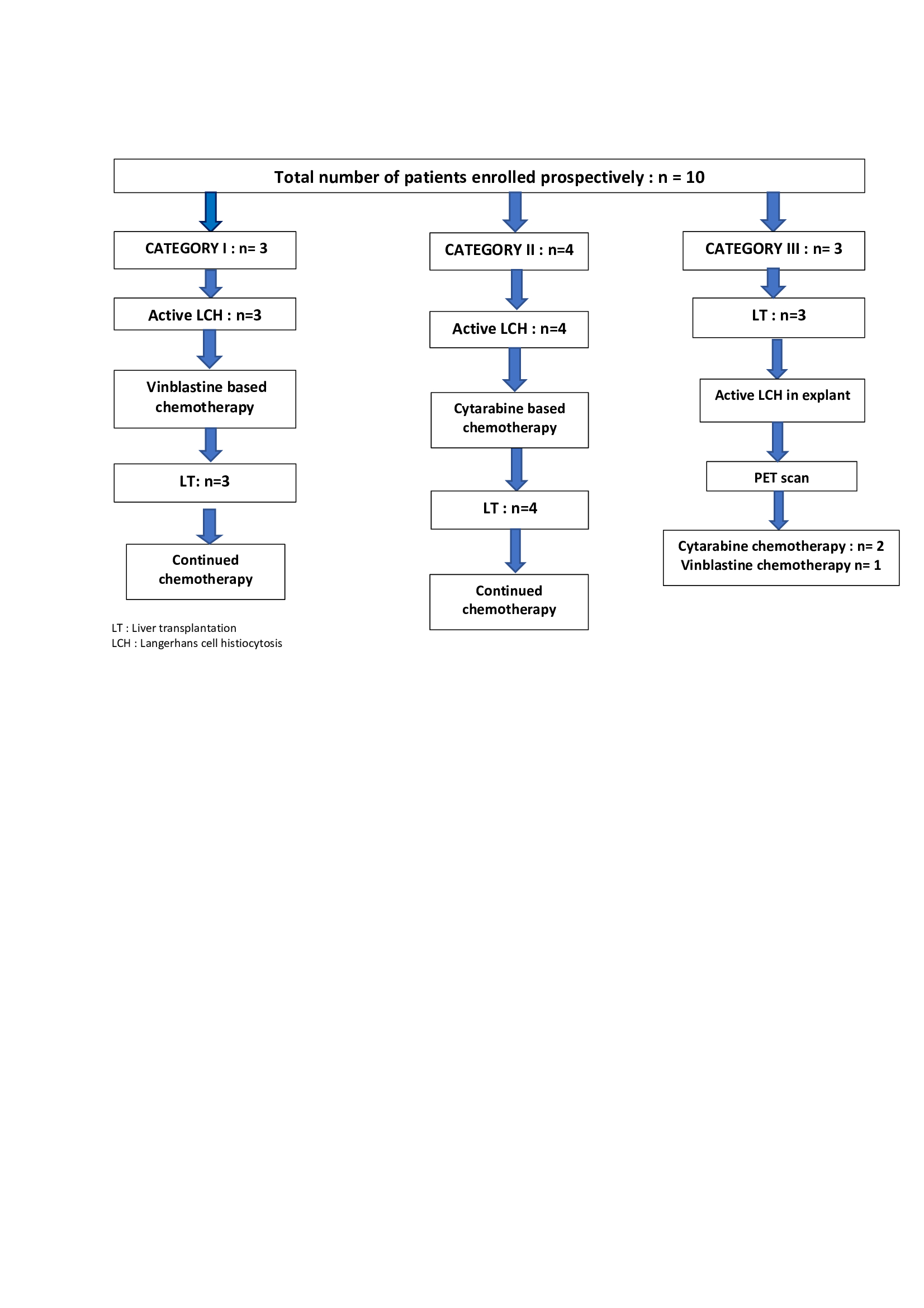 . Diagnosis was made in the pre LT period in 7 and post LT from the explant liver in 3 children, whose pre LT working diagnosis were Caroli’s disease (n=2) and primary sclerosing cholangitis(PSC) (n=1). Category I had 3, category II had 4 and we introduced Category III for those diagnosed from explant (n=3).(Figure 1). Indications for LT included decompensation and/or advanced portal hypertension, intractable pruritus and it was performed at 41.5 (17-140) months of age. Explant was positive for V600E BRAF mutation in 3 patients. Chemotherapy was continued post LT as per protocol (Fig 2
. Diagnosis was made in the pre LT period in 7 and post LT from the explant liver in 3 children, whose pre LT working diagnosis were Caroli’s disease (n=2) and primary sclerosing cholangitis(PSC) (n=1). Category I had 3, category II had 4 and we introduced Category III for those diagnosed from explant (n=3).(Figure 1). Indications for LT included decompensation and/or advanced portal hypertension, intractable pruritus and it was performed at 41.5 (17-140) months of age. Explant was positive for V600E BRAF mutation in 3 patients. Chemotherapy was continued post LT as per protocol (Fig 2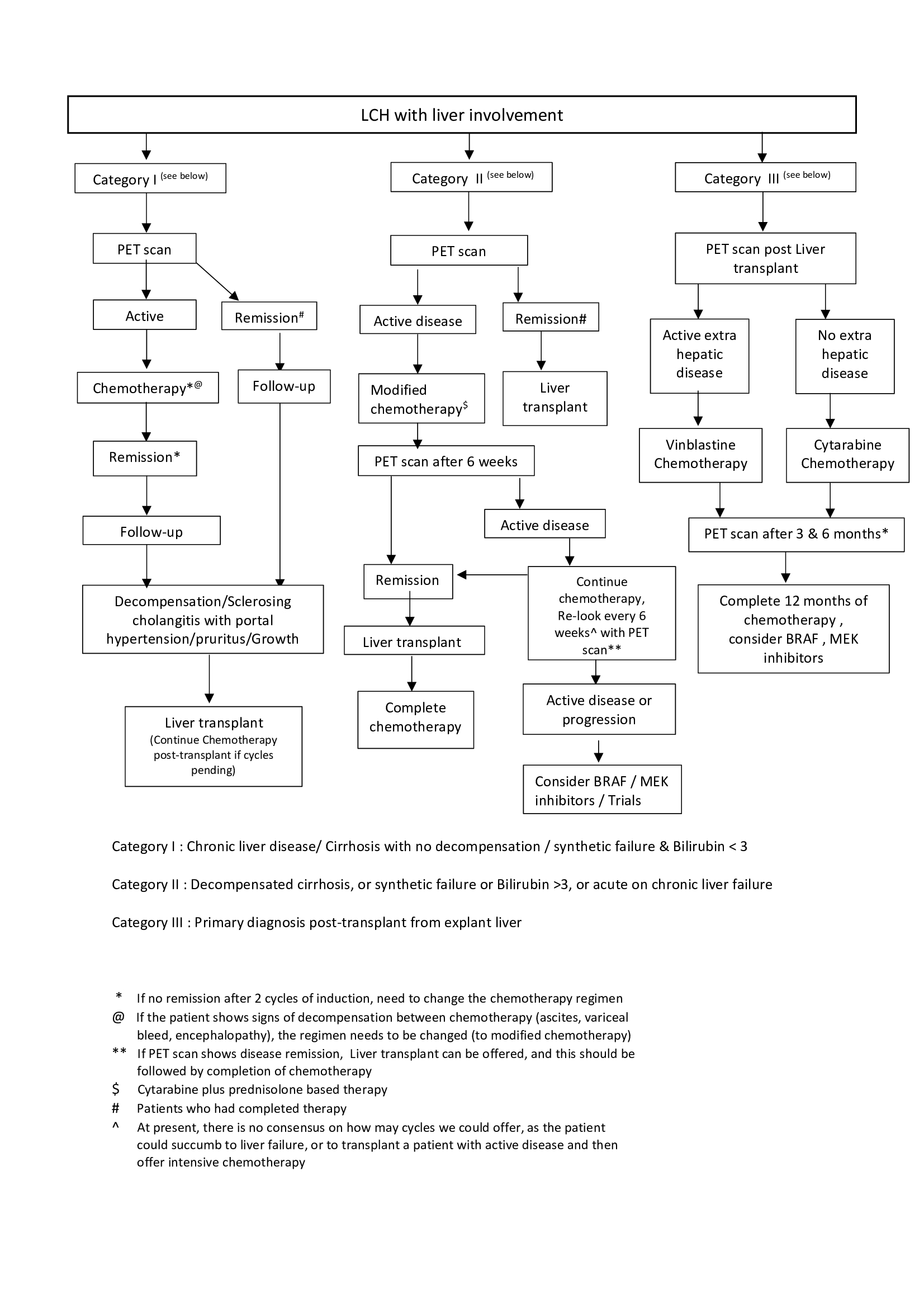 ). Trametinib was started for 2 and Vemurafenib for 1. After a median follow of 18 (4-28) months, 9 patients are alive with normal graft function. None of these patients had rejection, lymphoproliferation or disease recurrence in the graft.
). Trametinib was started for 2 and Vemurafenib for 1. After a median follow of 18 (4-28) months, 9 patients are alive with normal graft function. None of these patients had rejection, lymphoproliferation or disease recurrence in the graft.
Conclusion : The previously proposed algorithm based approach in patients with LCH ensures excellent post LT outcomes. LCH can be a mimicker of conditions like Caroli’s disease or PSC.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00