Pediatric ABO-incompatible liver transplant outcomes using intravenous immunoglobulin are effective and comparable to plasmapheresis desensitization
Katie Conover1,2, Lauren Maloney2, Catherine A. Chapin3, Riccardo Superina4, Megan Adams2, Michael Wachs2, Amy Feldman1,2, Sarah A. Taylor1,2.
1Department of Pediatrics, University of Colorado, Aurora, CO, United States; 2Department of Pediatrics, Northwestern University, Feinberg School of Medicine, Ann & Robert H. Lurie Children's Hospital, Chicago, IL, United States; 3Division of Transplant and Advanced Hepatobiliary Surgery, Northwestern University, Feinberg School of Medicine, Chicaog, IL, United States; 4Section of Pediatric Gastroenterology, Hepatology, and Nutrition, Digestive Health Institute, Children's Hospital Colorado , Aurora, CO, United States
Introduction
ABO-incompatible (ABOi) grafts increase the donor pool and reduce pediatric liver transplant (LT) waitlist mortality. Desensitization prevents LT complications but centers have wide variability in protocols.
Methods
We performed a retrospective review of ABOi LTs receiving peri-operative IVIG desensitization at Center 1 from 2015 to 2023. Patients were matched to ABO-compatible (ABOc) LTs by age, diagnosis, year of transplant and listing status. ABOi LT outcomes were compared to Center 2 (plasmapheresis desensitization); centers used similar immunosuppression regimens. Chi-squared, Mann-Whitney U, and Kaplan-Meier survival analysis were performed.
Results
Twelve patients received ABOi LT (median age 7.5 months). Three reached anti-AB IgG>1:8 or IgM>1:32; 1 required rituximab. Comparing ABOi versus ABOc patients showed similar use of living donor LT (p=0.41) and technical variant grafts (p=0.65). There were no significant differences in graft or patient survival, hepatic artery thrombosis, portal vein thrombosis, biliary leak or stricture (p>0.05).
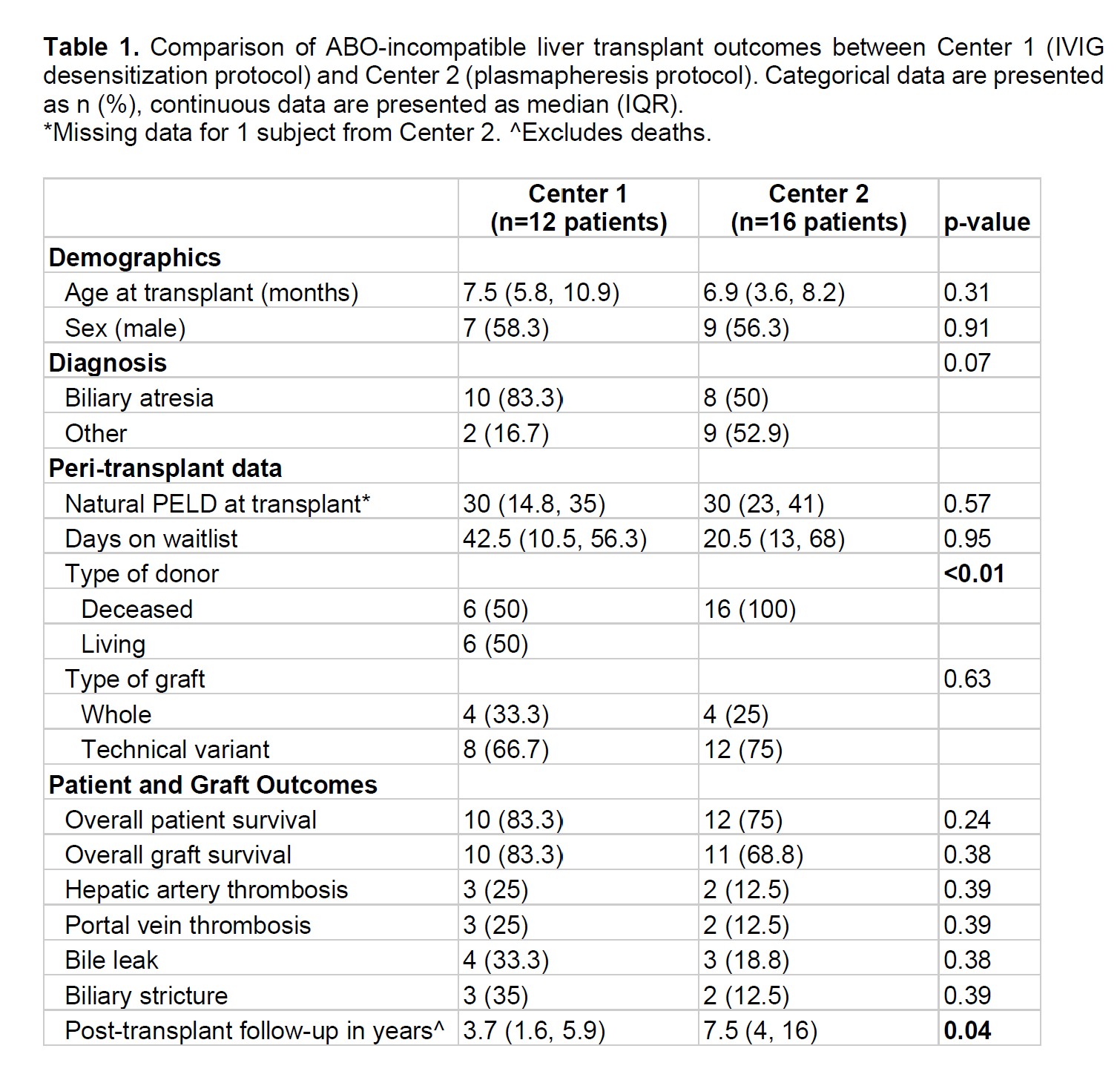
Comparing ABOi LTs between centers showed no significant differences in age, waitlist time, or natural PELD (p>0.05). Use of technical variant grafts was >50% at both centers. Center 1 had more living donor LT (p<0.01). There were no significant differences in graft or patient survival, vascular or biliary complications (p>0.05; Table 1).
Discussion
Matched ABOi and ABOc pediatric LT recipients had similar graft and patient outcomes using IVIG desensitization for ABOi LTs. Furthermore, ABOi outcomes were similar using IVIG and plasmapheresis desensitization. Prospective studies controlling for center-to-center differences are needed to optimize ABOi desensitization and decrease pediatric LT waitlist mortality.
Lauren Pratscher.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00