Evaluation of ddcfDNA Thresholds in Paediatric Liver Transplant Recipients via Serial Monitoring
Naresh Shanmugam1, Anu Vasudevan1, Mohamed Rela1, Avinash Ramani2, Bhavani Gunasekaran2, Pavan kumar K Bapatla2, Maharani Ramamoorthi2, Nivethitha Jayakanthan2, Agragesh Ramani2.
1The Institute of Liver Disease and Transplantation, Dr.Rela Institute and Medical Centre, Chennai, India; 2Molecular Biology, Acrannolife Genomics Private Limited, Chennai, India
Aim:
This study aims to evaluate donor-derived cell-free DNA (ddcfDNA) levels in paediatric liver transplant recipients through serial monitoring, assessing its potential as a non-invasive biomarker for graft health, rejection detection, and early intervention.
Materials and Methods:
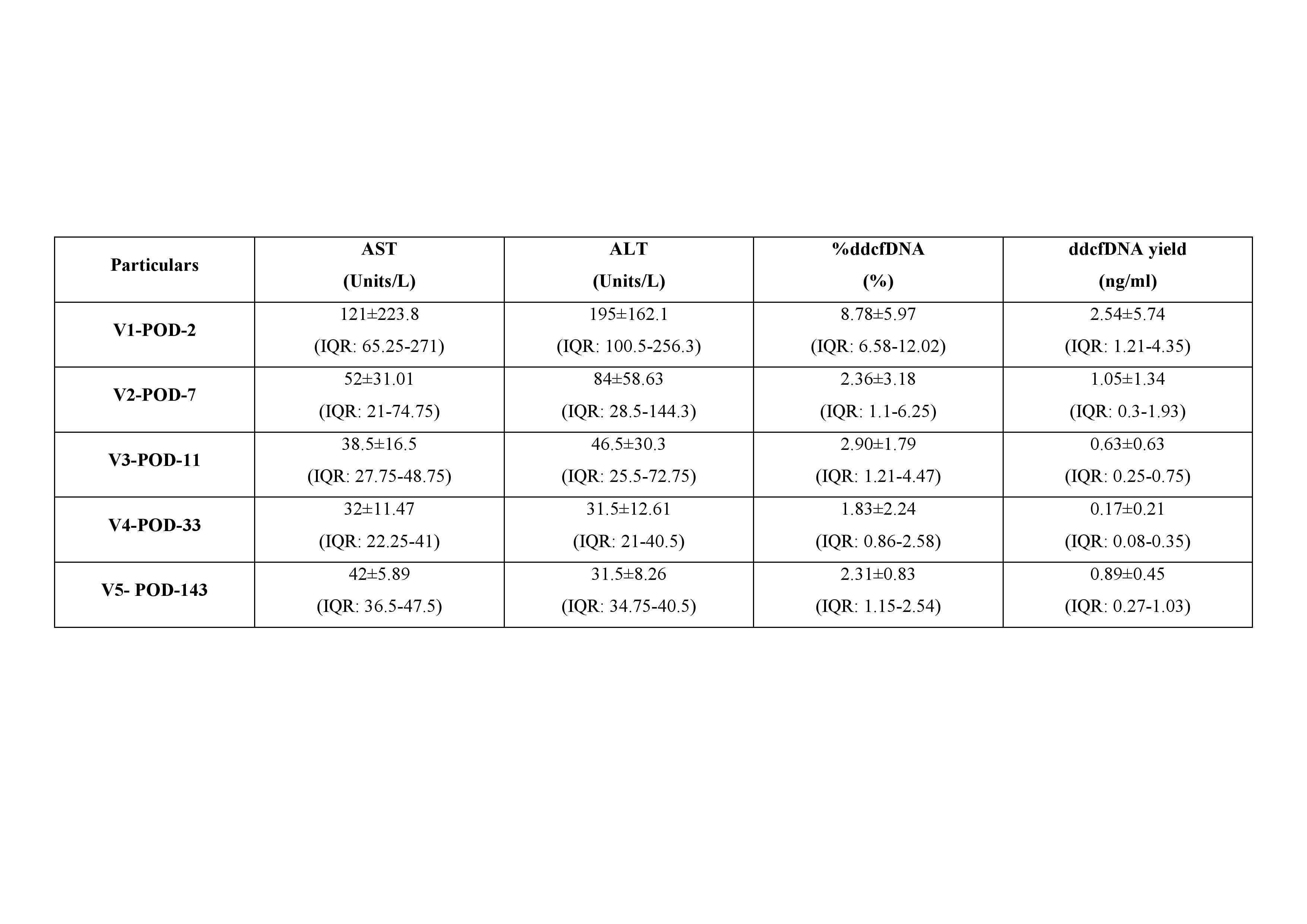
The ddcfDNA levels were monitored at five specific time points post-transplant in a cohort of stable pediatric liver transplant recipients with declining transaminases. Patients who experienced a sudden increase in liver function tests (LFTs) and were suspected of having rejection were excluded from the study. Blood samples were collected at regular intervals post-transplant: (V1- 1-2 POD; V2- 7-8 POD; V3- 10-14 POD; V4- 30 POD; D5- Follow-up) to measure ddcfDNA levels. Missing a protocol visit was not an exclusion criteria. ddcfDNA levels was measured using a patented Trunome® GrafAssure™ Test as described in Jana et al., 2024. Clinical data and graft function markers were used for correlation analysis. Statistical methods were employed to achieve the baseline levels of ddcfDNA as an indicator of graft health compared to biochemical markers and clinical findings.
Results and Discussion:
A total of 23 paediatric subjects who underwent liver transplant with stable graft function through out the study was analysed. The median ddcfDNA% values for the LFT’s and ddcfDNA levels were derived and are represented in Table 1. High ddcfDNA levels were observed during initial days after transplant and were gradually declined to the levels of 2.36% by 7th POD and were maintained low for the rest of the observation period. The initial elevation of the ddcfDNA% is presumably from the ischemia/reperfusion damage (8.78%). On the other hand, LFT levels were elevated in the initial days post transplant and were stabilized gradually by 11th POD unlike ddcfDNA levels which were reached to baseline by 7th POD. Therefore, ddcfDNA levels might act as early predictor of graft health than LFTs.
Conclusion:
The study establishes ddcfDNA baseline levels post liver transplant in paediatric subjects to guide clinical decisions and the potential for personalizing transplant care based on real-time molecular data. Serial monitoring allows for timely intervention, potentially reducing the need for invasive biopsies and improving patient outcomes.
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00