
Impact of induction therapy on rejection in pediatric transplantation: A multicenter study in the United States
Tetsuya Tajima1, Clifford Chin2, Dev Desai3, Thomas M Fishbein4, George V Mazariegos5, Akin Tekin6, Robert S Venick7, Kazunari Sasaki1, Sheri M Krams1, Olivia M Martinez1, Carlos O Esquivel1.
1Department of Surgery, Division of Abdominal Transplantation, Stanford University School of Medicine, Stanford, CA, United States; 2Department of Pediatrics and Cincinnati Children’s Hospital, University of Cincinnati, Cincinnati, OH, United States; 3Phoenix Children’s hospital, Phoenix, AZ, United States; 4Departments of Surgery and Pediatrics, MedStar Georgetown University Hospital, Washington, DC, United States; 5Department of Pediatrics, University of Pittsburgh Medical Center (UPMC) Children’s Hospital, Pittsburgh, PA, United States; 6Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, United States; 7Department of Pediatric Gastroenterology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, United States
Clinical Trials in Organ Transplantation in Children (CTOTC-06).
Introduction: Induction therapy is intensive immunosuppression administered at transplant to mitigate the risk of rejection; however, the optimal induction therapy for pediatric transplantation remains unknown.
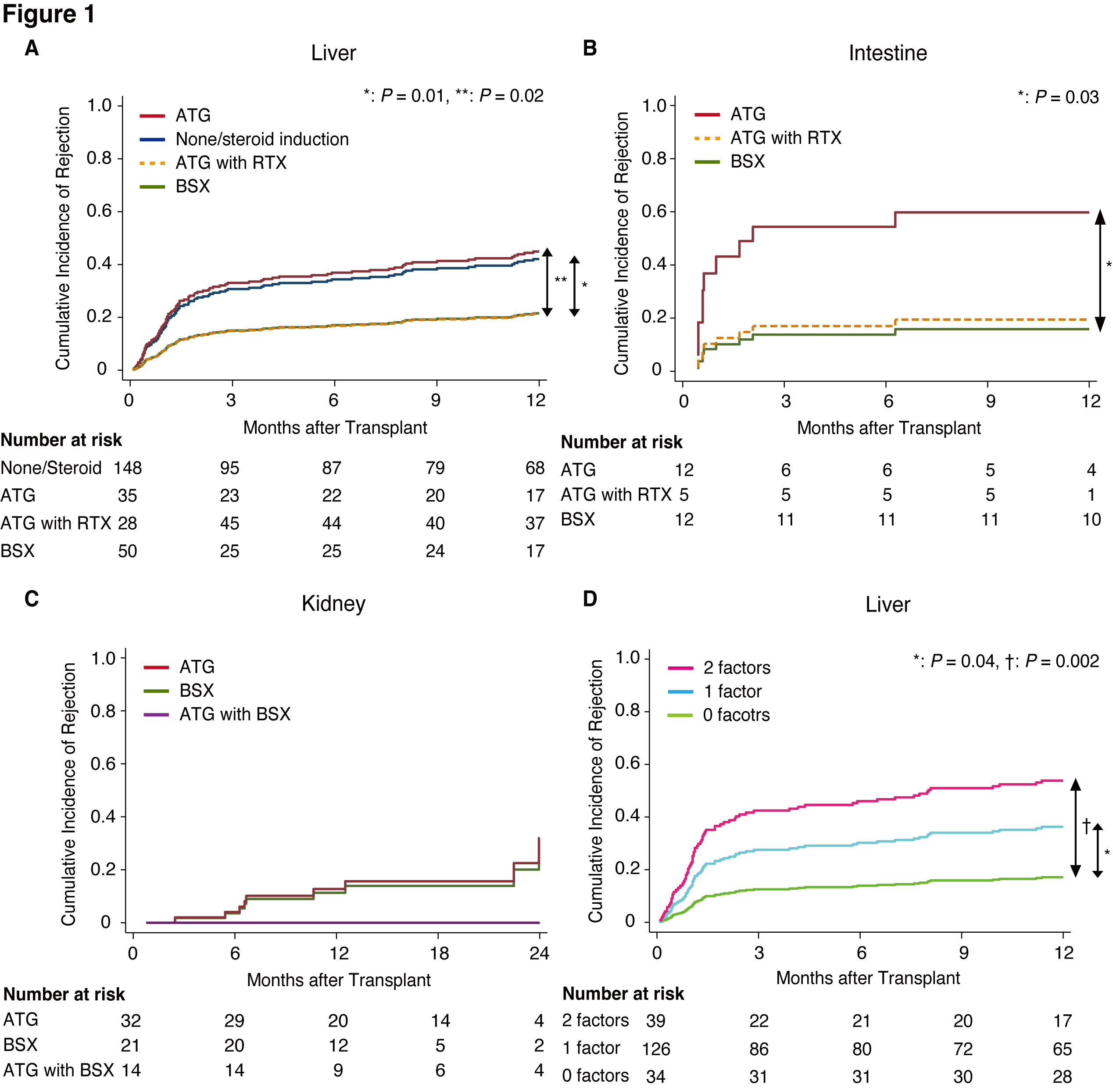
Methods: This prospective, multicenter, study (NCT02182986) enrolled 944 children (≤21 years), of whom 872 underwent liver, heart, kidney, intestinal, or multivisceral transplants (LT, HT, KT, and IT, respectively) across seven U.S. centers between 2014 and 2019. Following patient selection, 470 patients (261 LTs, 97 HTs, 79 KTs, and 33 ITs) were retrospectively analyzed. Clinical variables included sex, age, ethnicity, pre-transplant Epstein-Barr virus (EBV) and cytomegalovirus serologies, and immunosuppression. Rejection incidence was evaluated using cumulative incidence functions, with patient mortality as a competing risk.
Results: In the LT cohort, ATG vs. basiliximab (SHR: 2.83 [1.19–6.71], P=0.02) and non/steroid-based induction vs. basiliximab (2.63 [1.27–5.47], P=0.01) were significant risk factors for rejection (Figure-1A). ATG vs. basiliximab was also a significant risk factor for rejection post-IT (5.74 [1.11–29.8], P=0.04, Figure-1B). In KT, no rejection was observed within two years post-transplant in patients receiving both ATG and basiliximab (Figure-1C). For HT, EBV D+R- serostatus was a significant risk factor for rejection (2.02 [1.06–3.84], P=0.03) and was similarly associated with rejection risk in the univariable analysis of LT recipients. Rejection risk in LT was significantly stratified by the combination of induction therapy and EBV D+R- serostatus (Figure-1D).
Conclusion: Basiliximab was superior to ATG, with significantly lower rejection rates in LT and IT. The additive effect of EBV D+R- serostatus and induction therapy affected the incidence of rejection in pediatric LT.
NIH UO1 AI104342. UM2AI117870 (Rho Federal Systems). Stanford Transplant and Tissue Engineering Center of Excellence fellowship. Overseas Research Fellowships of the Japan Society for the Promotion of Science (JSPS).
The WebApp is sponsored by:

If you have any questions during the meeting, please go to the registration desk. Our emails will be monitored sporadically.
REGISTRATION DESK OPENING TIMES
Thursday, May 1, 2025, 07:00-17:30 Friday, May 2, 2025, 07:00-12:00